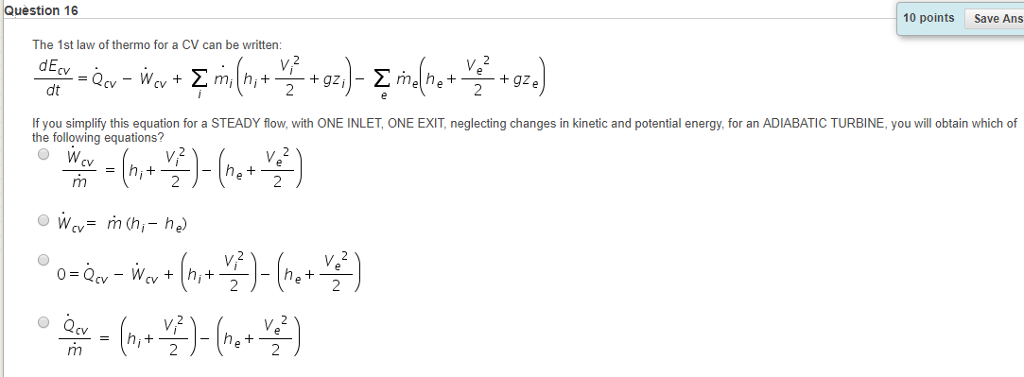Kinesis CV-TSP-507 Outlet Check Valve Assembly, Thermo Scientific Surveyor LC; 1/EA from Cole-Parmer

Thermo Scientific™ Duke Standards™ 2000 Series Uniform Polymer Particles Dia.: 14.6μm ± 0.5μm, σ = 1.1μm, 7.5% CV Thermo Scientific™ Duke Standards™ 2000 Series Uniform Polymer Particles | Fisher Scientific

Modeling of Thermo-Electro-Mechanical Manufacturing Processes eBook by Niels Bay - EPUB | Rakuten Kobo United States

Thermodynamic study (a) Effect of temperature on the CV adsorption onto... | Download Scientific Diagram
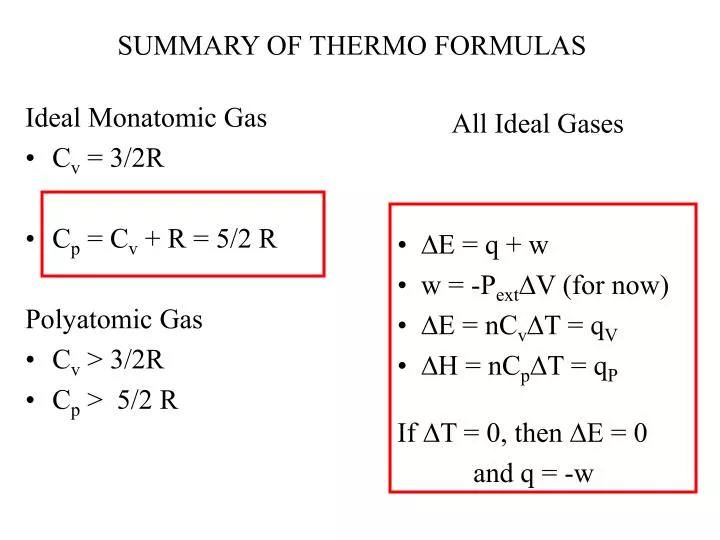
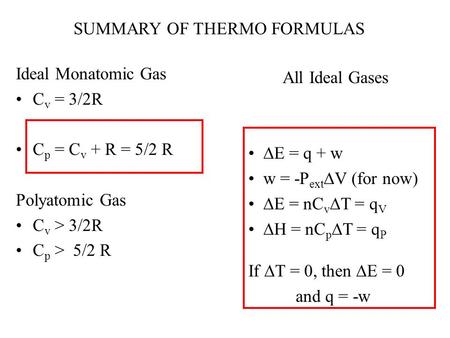
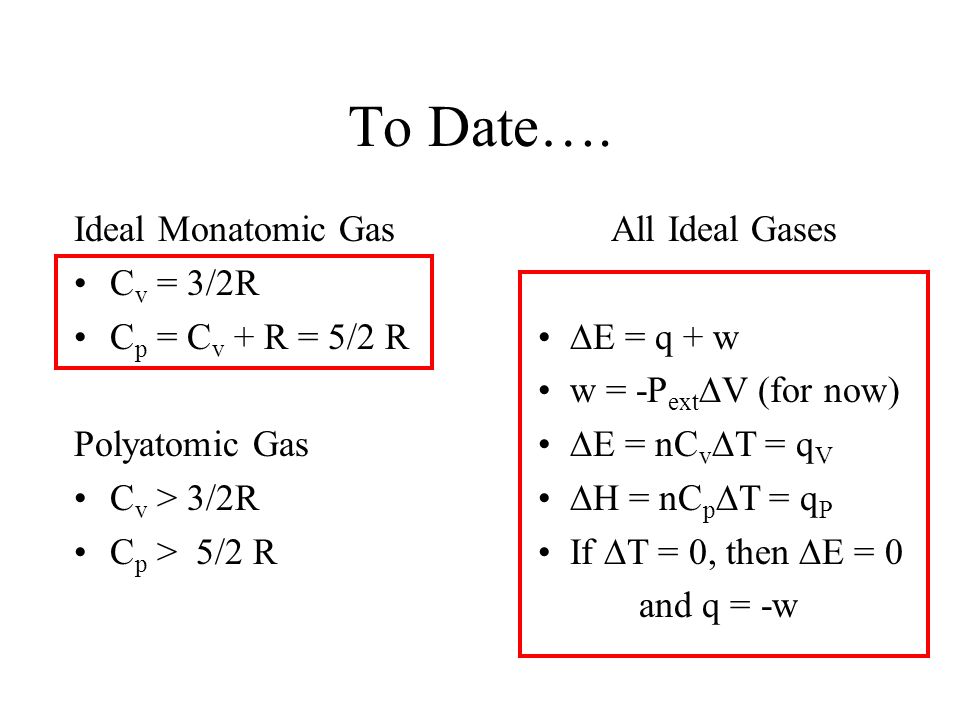
PPT - Ideal Monatomic Gas C v = 3/2R C p = C v + R = 5/2 R Polyatomic Gas C v > 3/2R C p > 5/2 R PowerPoint Presentation - ID:4355470
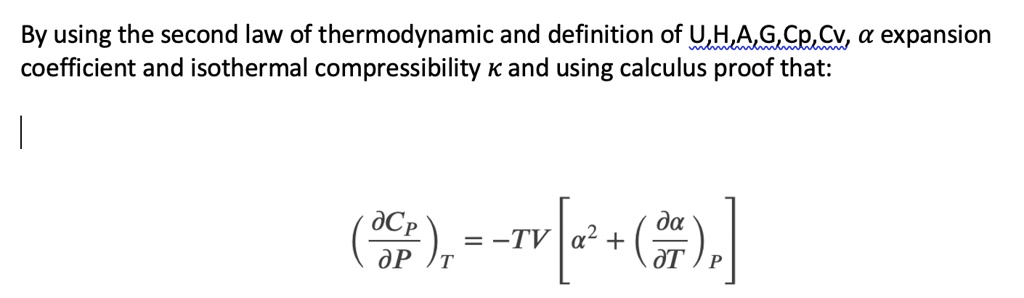
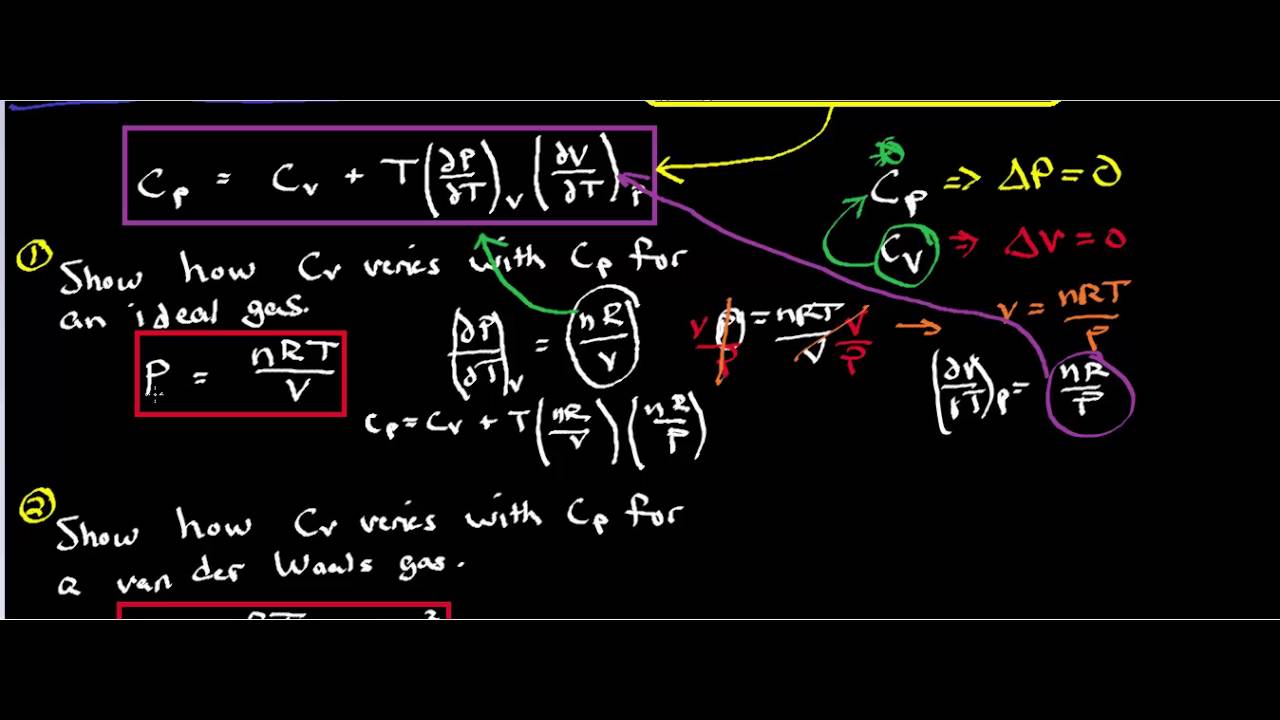
SOLVED: By using the second law of thermodynamic and definition of U,HAG,Cp, Cv a expansion coefficient and isothermal compressibility K and using calculus proof that: aCp da = TV 02 + dP T

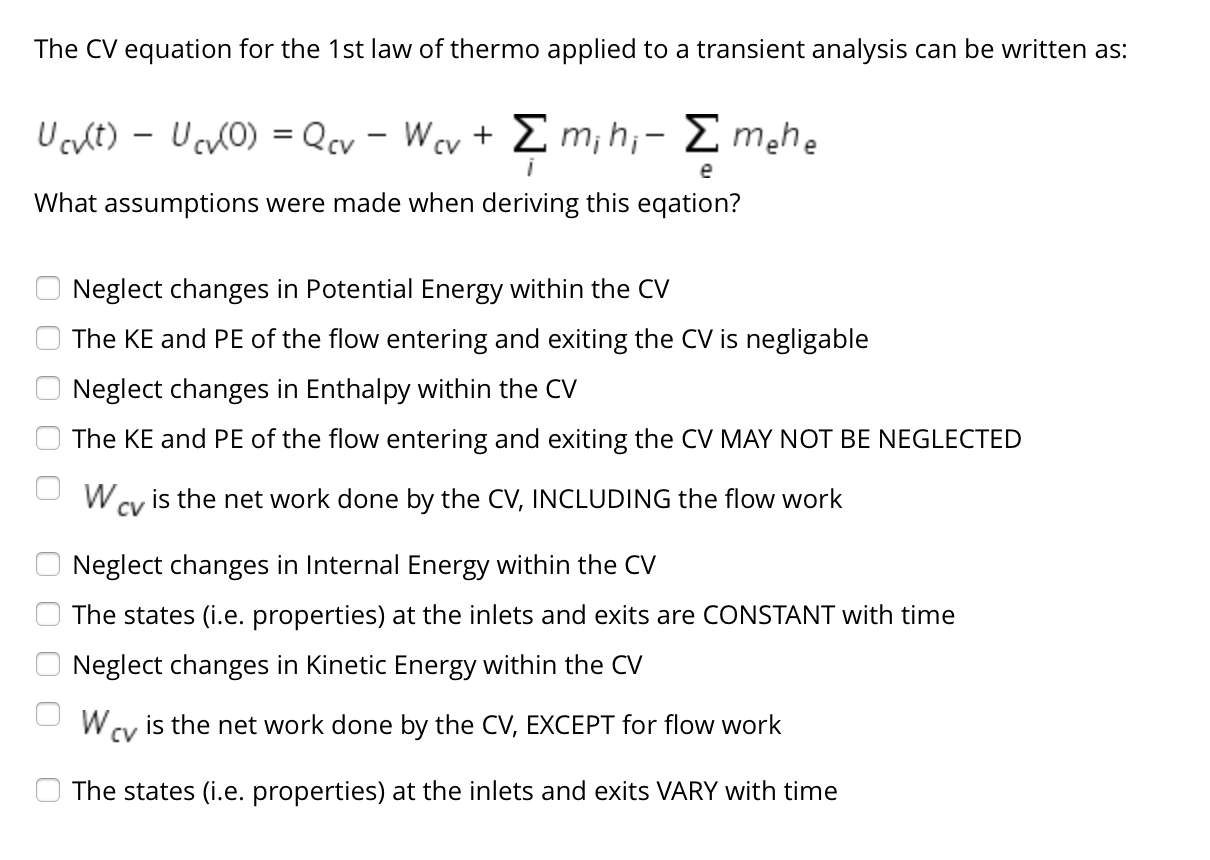
SOLVED: The Ist law of thermodynamics for CV can be written: dEcv Qcv Wcv Em(+V+gz)- Emha 9ze) If you simplify this equation for a STEADY flow; with ONE INLET, ONE EXIT neglecting

Thermo Scientific™ 7000 Series Copolymer Microsphere Suspensions 17μm; CV ≤ 16%; 1000mL Thermo Scientific™ 7000 Series Copolymer Microsphere Suspensions | Fisher Scientific